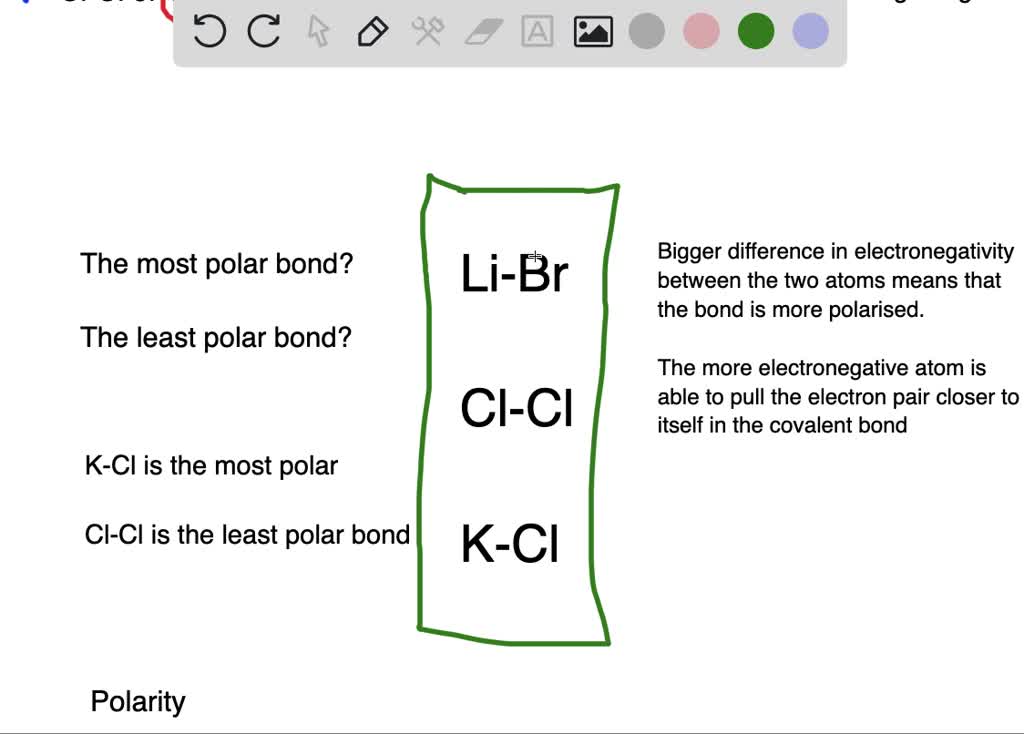
Which Bond Is Least Polar . Electronegativity difference must be less than 0.5. Recall that for a covalent bond to be:
Solved Which Bond Is Least Polar? H-C H-Si H-N H-P | Chegg.com from www.chegg.com
As they are two of the same atom, they will have no electronegativity difference, making the bond nonpolar. (the difference in the electronegativies of the two atom is least)
Solved Which Bond Is Least Polar? H-C H-Si H-N H-P | Chegg.com
Here are the electronegativity values. When differences are 1.7 or greater, the bond is usually ionic. C − h bond is least polar as the electronegativity difference between c and h is the least.
Source: chemistrysaaangus.weebly.com
And less than 0.5 are considered to be nonpolar. Therefore, it is the least polar. This phenomenon arises when there lies no difference in the electronegativities of the two combining atoms.
Source: www.chegg.com
The least polar bond is the one with the least difference in electronegativity between the two elements. A bond that is formed by the equal sharing of electrons between the combining atoms is called a nonpolar covalent bond. Bond polarity can be calculated, examining the pauling scale electronegativity values of the two atoms.
Source: www.youtube.com
When the difference in electronegativity between the atoms is zero, what do we call the bond? So that means the cf bond is the least polar. Here are the electronegativity values.
Source: www.chegg.com
As they are two of the same atom, they will have no electronegativity difference, making the bond nonpolar. This organic chemistry video tutorial explains how to determine which bond is more polar. So 2.55 then we have 1.9 and then we have gallium, which is at 1.81 and the sodium flooring bond is the most polar because it's sodium has.
Source: www.chegg.com
C−h bond is least polar as the electronegativity difference between c and h is the least. So let's just discuss polarity in a bit more detail before doing so. It also explains how to rank the bonds from least polar to most po.
Source: www.clutchprep.com
Now clearly the difference in electronegativities is minimum between sulphur and hydrogen. Therefore, it is the least polar. To find which one of the bond is least polar you need to find the electronegativity difference between the atoms that are involved in bonding.the more is the electronegativity difference (∆en) the more is the bond polarity and vice versa.
Source: slideplayer.com
You need to draw the lewis electron dot structure and assign a sh… view the full answer In a polar bond, the electrons are not equally distributed between the atoms. The higher the electronegativity difference, the more polar a bond is.
Source: www.clutchprep.com
Bond polarity can be calculated, examining the pauling scale electronegativity values of the two atoms. This higher the difference, the more polar the bond is. When differences are 1.7 or greater, the bond is usually ionic.
Source: www.chegg.com
They will be the two elements which have electronegativities that are closest. The least polar bond is the one with the least difference in electronegativity between the two elements. A bond that is formed by the equal sharing of electrons between the combining atoms is called a nonpolar covalent bond.
Source: www.youtube.com
Increasing the electronegativity difference lead to a more polar bond. As they are two of the same atom, they will have no electronegativity difference, making the bond nonpolar. Therefore, it is the least polar.
Source: slideplayer.com
The least polar bond is found in a molecule of 1. Bond polarity can be calculated, examining the pauling scale electronegativity values of the two atoms. That would be carbon and iodine.
Source: www.bartleby.com
We've got l i b r, followed by clc out, followed by casey l. So we're gonna be identifying the most polar bond and the least polar bonds. Less than 1.7, the bond is usually covalent, less than 0.5 the bond has some degree of polarity.
Source: socratic.org
Polarity of substance is depend on difference of electronegativity values between bonded. The least polar bond would be between atoms have have the smallest difference in electronegativity. One way to think of a polar bond is that it is a bond in which the two atoms have sufficiently different electronegativities (one atom wants electrons more than the.
Source: www.chegg.com
It also explains how to rank the bonds from least polar to most po. The least polar bond would be between atoms have have the smallest difference in electronegativity. In a polar bond, the electrons are not equally distributed between the atoms.
Source: www.chegg.com
And then we're followed by s i. And less than 0.5 are considered to be nonpolar. C−h bond is least polar as the electronegativity difference between c and h is the least.
Source: chemistry.stackexchange.com
Polarity of substance is depend on difference of electronegativity values between bonded. The lower the difference in electronegativity between the bonded atoms, the less polar the bond. How do you tell which covalent bond is most polar?
Source: www.numerade.com
The higher the electronegativity difference, the more polar a bond is. So the bigger difference in election negativity between the two atoms are bonded means that the bond is more polarized. Less than 1.7, the bond is usually covalent, less than 0.5 the bond has some degree of polarity.
Source: www.numerade.com
In general, elements closer to one another on the periodic table will form bonds with less polarity. The difference between these values will determine the predominant type of bond between the respective atoms. It also explains how to rank the bonds from least polar to most po.
Source: chemistrytalk.org
So, d is the correct answer. The lower the difference in electronegativity between the bonded atoms, the less polar the bond. From the calculated en difference, the least polar is the bond between hydrogen and boron, a.
Source: www.numerade.com
Less than 1.7, the bond is usually covalent, less than 0.5 the bond has some degree of polarity. How do you tell which covalent bond is most polar? To find which one of the bond is least polar you need to find the electronegativity difference between the atoms that are involved in bonding.the more is the electronegativity difference (∆en) the.