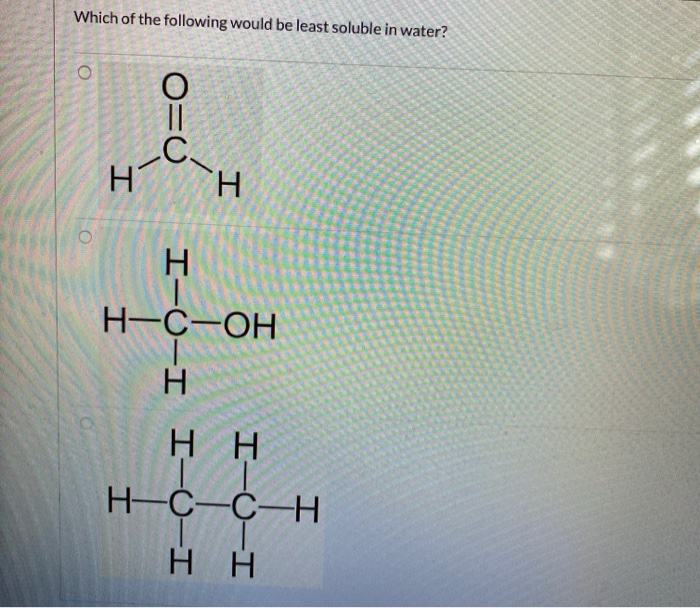
Which Of The Following Is Least Soluble In Water . A)nacl b)kcl c)nh4cl d)hcl 7.according to reference table g, which of the following substances is least soluble in 100 grams of water at 50ºc? Hexane, ch3ch2ch2ch2ch2ch3, is a hydrocarbon, and nonpolar.
Which Is Least Soluble In Water? - Youtube from www.youtube.com
So, agi is least soluble of the given options. Which compound is the least soluble in water?
Which Is Least Soluble In Water? - Youtube
H1 which of the following will be most soluble in water at 2 q c? So, agi is least soluble of the given options. Mgs (s) + 2h2o (l) = mg(oh)2 (s) + h2s (g) sodium salts are generally soluble in water.
Source: www.toppr.com
• view available hint (s) reset help propanol methane. Sodium chloride is the least soluble in which of the following liquids? Which one of the following is least soluble in water?
Source: www.toppr.com
Why baso4 is not soluble in water? Which is least soluble in water among the following? Ozone is used for purifying water because.
Source: www.youtube.com
A)nacl b)kcl c)nh4cl d)hcl 7.according to reference table g, which of the following substances is least soluble in 100 grams of water at 50ºc? Which is least soluble in water among the following? Now, we take each organic compound type to study their solubility in water.
Source: www.chegg.com
Which one of the following is least soluble in water? Except lithium hydroxide,all the alkali metal hydroxides are highly soluble in water.lioh is much less soluble on account of high lattice enthalpy. Mgs is easily decomposed (hydrolysed) by water to form insoluble magnesium hydroxide and hydrogen sulphide gas.
Source: www.chegg.com
Ozone is used for purifying water because. A)nacl b)kcl c)nh4cl d)hcl 7.according to reference table g, which of the following substances is least soluble in 100 grams of water at 50ºc? View available hints) reset help magnesium chlonde 1.
Source: www.coursehero.com
Therefore, for any compound to become soluble in water, it should be polar or have hydrogen bonding in it. All are soluble in water except mgs, magnesium sulphide. Which compound is the least soluble in water?
Source: www.youtube.com
Alcohols, carboxylic acids, carboxylic acid chlorides, amines, esters are usually soluble in water. To rank items as equivalent, overlap them. Rank from most to least soluble in water.
Source: employees.csbsju.edu
So,they are must soluble in water.nh3 is a polar covalent compound which is soluble in polar solvent water. 6.which compound becomes less soluble in water as the temperature of the solution is increased? All are soluble in water except mgs, magnesium sulphide.
Source: www.bartleby.com
Mgs is easily decomposed (hydrolysed) by water to form insoluble magnesium hydroxide and hydrogen sulphide gas. Among the following compounds, the strontium fluoride has the least solubility. Check answer and solution for a
Source: neetlab.com
Ozone is used for purifying water because. Alcohols, carboxylic acids, carboxylic acid chlorides, amines, esters are usually soluble in water. Rank from most to least soluble in water.
Source: www.chegg.com
6.which compound becomes less soluble in water as the temperature of the solution is increased? Which is least soluble in water among the following? Which of the following is least soluble in water ?
Source: www.clutchprep.com
Due to lack of hydrogen bonding, alkane is least soluble inter. Zn (s) + ni2+ (aq) zn2+ (aq) + ni (s) the emf generated by the cell when ni2+ = 0.100 m and zn2+ = 2.25 m is a) 0.56 b) 0.50 c) 0.44 On adding water in barium sulphate its hydration energy decreases more rapidly in comparison to its.
Source: www.youtube.com
The large hydrocarbon part is hydrophobic and thus there are low chances of solubility. (a) (ch3)2nh (b) c6h5nh2 (c) ch3nh2 (d) (ch3)3n. 1) acetone 2) formaldehyde 3) acetaldehyde 4) benzaldehyde please explain the answer also thank you
Source: www.clutchprep.com
Which compound is the least soluble in water? Which one of the following is least soluble in water? Rank the following organic compounds from most soluble in water to least soluble in water.
Source: www.numerade.com
On adding water in barium sulphate its hydration energy decreases more rapidly in comparison to its lattice energy. Chlorine has electron donating resonance ( + r) effect. Answer the following questions based on the solubility curve below.
Source: www.chegg.com
Thus, the least soluble compound is hgs. On the right here we question: Sodium chloride is the least soluble in which of the following liquids?
Source: www.bartleby.com
Among the following compounds, the strontium fluoride has the least solubility. Which of the following is least soluble in water ? Rank the following organic compounds from most soluble in water to least soluble in water.
Source: www.doubtnut.com
A) ch3oh b) ch3ch2ch2oh c) ch3ch2oh d) ch3ch2ch2ch2oh e) ch3ch2ch2ch2ch2oh question 2 the standard emf for the cell using the overall cell reaction below is +0.48 v: On adding water in barium sulphate its hydration energy decreases more rapidly in comparison to its lattice energy. Which of the following is least soluble in water at 298 k?
Source: brainly.com
Which one of the following substances is least soluble in water? H1 the least soluble salt in water is. A)nacl b)kcl c)nh4cl d)hcl 7.according to reference table g, which of the following substances is least soluble in 100 grams of water at 50ºc?
Source: www.embibe.com
Which is the least soluble compound? A)nacl b)kcl c)nh4cl d)hcl 7.according to reference table g, which of the following substances is least soluble in 100 grams of water at 50ºc? Rank the following substances in order from most soluble in water to least soluble in water: