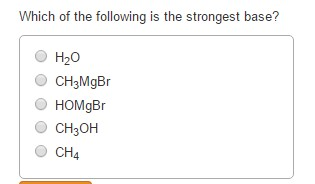Which Of The Following Is The Strongest Base . Which of the following is the strongest base: Also according to the fajans rule greater the size of the atom from the hydroxyl group stronger is the base.
Solved Which Of The Following Is The Strongest Base? O | Chegg.com from www.chegg.com
The water is acting as an acid, and its conjugate base is the hydroxide ion. There are many concept which can define a base.
Solved Which Of The Following Is The Strongest Base? O | Chegg.com
The base is followed by its kb value. Koh is stronger base because the ion of potassium is larger than na ion.larger the size more is the distance between the two molecule easier is to dissociate them Which of the following is the strongest base?
Source: www.youtube.com
10 which of the following is the strongest brønsted base? E) none of the choices is correct. Hence, the correct answer is option is b.
Source: www.sarthaks.com
Which of the following is the strongest base? Complete the following reaction and identify the brønsted base:naoh(aq) + hcl(aq) → a. And we know that strong bases remove proton from very weak acids hence with this we can conclude that that correct answer among all the options is c h 3 n h 2 as we know that it is.
Source: www.chegg.com
E) none of the choices is correct. Which of the following aniline derivatives is the strongest base? Meo o e.cl question 8 the stronger the acid, the higher the pka and the lower the ka o true o false question 9 which of the following is the strongest base?
Source: www.chegg.com
Which of the following is the strongest base? Hence, the correct answer is option is b. Therefore, the strongest base is ro− and the correct option is (b).
Source: www.sarthaks.com
If so, indicate the formulas of the ions in. Which of the following is the strongest base in aqueous solution? Which of the following is strongest base :
Source: oneclass.com
The reducing character of hydrides increases down the group due to decrease in bond dissociation enthalpy. Which of the following is the strongest base? Which of the following is strongest base :
Source: www.toppr.com
Koh is stronger base because the ion of potassium is larger than na ion.larger the size more is the distance between the two molecule easier is to dissociate them Asked jul 30, 2019 in chemistry by rakhee jain ( 88.1k points) Which of the following compounds is most basic?
Source: www.toppr.com
The base is followed by its kb value. Roh is the acid of ro− conjugate base, hoh is the acid of −oh,c6h5oh is the acid of c6h5o− and ho is the acid of. 11 dibutylamine, (c 4 h 9 ) 2 nh, and anisole, c 6 h 5 och 3 , have similar boiling points, and are relatively insoluble in.
Source: www.chegg.com
Which of the following bases is the strongest? How might a mixture of these compounds be separated into the pure components? Which of the following is strongest base :
Source: www.toppr.com
Knh2 +m nh2 m (b) meo +m nh tu nh, lum (d) c) on 86. (a)ammonia is the weakest reducing agent and the strongest base among group 15 hydrides. Which of the following is strongest base :
Source: chemistry.stackexchange.com
Which of the following is strongest base : Which of the following bases is the strongest? C 6 h 5 n h c h 3 is less basic than c h 3 n h 2 and more basic than n c − c h 2 n h 2.thus, the strongest base in aqueous medium is (c h 3 ) 2.
Source: www.numerade.com
Which of the following is the strongest base? Consider the following compounds and answer a and b. (d)ammonia is the strongest reducing agent as well as the weakest base among group 15 hydrides.
Source: www.doubtnut.com
Knh2 +m nh2 m (b) meo +m nh tu nh, lum (d) c) on 86. A.iodide anion, i b.fluoride anion, f c.bromide anion, br d.chloride anion, cl Which one of the following is not a soluble, strong base?
Source: www.chegg.com
Which of the following is the strongest base? Consider the following compounds and answer a and b. 11 dibutylamine, (c 4 h 9 ) 2 nh, and anisole, c 6 h 5 och 3 , have similar boiling points, and are relatively insoluble in water.
Source: www.youtube.com
Which of the following is the strongest base: Among all these acids, roh is the weakest acid. Meo o e.cl question 8 the stronger the acid, the higher the pka and the lower the ka o true o false question 9 which of the following is the strongest base?
Source: www.youtube.com
Which of the following is strongest base : Which of the following is the strongest base in aqueous solution? Koh is stronger base because the ion of potassium is larger than na ion.larger the size more is the distance between the two molecule easier is to dissociate them
Source: www.doubtnut.com
So bases are those substance which can easily give oh ion. Which of the following is the strongest base? Which of the following is the strongest base?
Source: www.meritnation.com
Which response includes all of the following that are insoluble bases, and no others? Which of the following bases is the strongest? 11 dibutylamine, (c 4 h 9 ) 2 nh, and anisole, c 6 h 5 och 3 , have similar boiling points, and are relatively insoluble in water.
Source: www.vedantu.com
A) ch3coch3 b) ch3cooh c) nh3 d) h2o We should know that here in chemistry the base is the one which can either accept the hydrogen ions or it can donate the pair of valence electrons. A lewis acid is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a lewis base.
Source: actingcolleges.org
Which of the following compounds is most basic? The hydroxides of the group i (alkali metals) and group ii (alkaline earth) metals usually are considered to be strong bases.these are classic arrhenius bases.here is a list of the most common strong bases. The base is followed by its kb value.