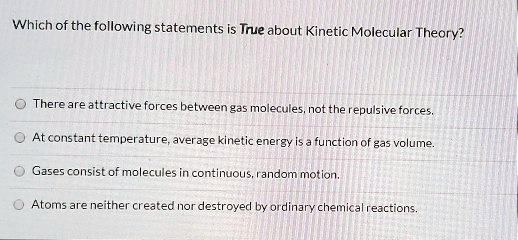
Which Statement Is True About Kinetic Molecular Theory . (4 points) matter consists of only large molecules. Matter is made up of only charged particles.
Chapter 5 Kinetic Molecular Theory Keave And Average Velocity from studylib.net
A) a single particle does not move in a straight line. B)gas molecules move rondmly in different directions,continuously colliding with.
Chapter 5 Kinetic Molecular Theory Keave And Average Velocity
B) the size of the particle is large compared to the volume. Their size is assumed to be much smaller than the average distance between the particles. The kinetic molecular theory of gases is a model that helps us understand the physical properties of gases at the molecular level.
Source: studylib.net
According to kinetic molecular theory (kmt), which statement is true? Which of the following statements about the kinetic particle theory is not true? An ideal gas differs from a real gas in that the molecules of an ideal gas _____.
Source: www.chegg.com
Kinetic energy is the energy of mass in motion. Which statement is true according to the kinetic theory? Which statement describes the particles of an.
Source: www.clutchprep.com
Quesliun which statement is true about kinetic molecular theory? The particles of matter have zero kinetic energy. The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior.
Source: www.doubtnut.com
1 📌📌📌 question which statement is true according to the kinetic theory? The molecules are close together. An ideal gas differs from a real gas in that the molecules of an ideal gas _____.
Source: www.numerade.com
The kinetic energy of an object is the energy it has because of its motion. The average kinetic energy of a collection of gas particles is directly proportional to absolute temperature only. The particles of matter are in constant motion.
Source: www.numerade.com
An ideal gas differs from a real gas in that the molecules of an ideal gas _____. According to kinetic molecular theory (kmt), which statement is true? Molecules of different gases with the same mass and temperature always have the same average volume.
Source: www.chegg.com
According to kinetic molecular theory,which of the following statements is true of the molecules in the gaseous state? Kinetic molecular theory states that gas particles are in constant motion and exhibit perfectly elastic collisions. Which statement is true according to the kinetic molecular theory?
Source: www.chegg.com
Which statement is true about kinetic molecular theory? Quesliun which statement is true about kinetic molecular theory? The kinetic molecular theory of matter states that:
Source: slideplayer.com
According to the kinetic molecular theory which statement describes the particles of an ideal gas; In the kinetic molecular theory of gas behavior, the assumption is made that gas molecules (a) move rapidly in random directions. Which statement about the molecules in the gas carbon dioxide is correct?
Source: www.numerade.com
Which statement is true about kinetic molecular theory?a) a single particle does not move in a straight line.b) the size of the particle is large compared to the volume.c) the collisions of particles with one another is completely elastic.d) the average kinetic energy of a particle is not proportional to the temperature. Matter is made up of only charged particles..
Source: www.clutchprep.com
The average kinetic energy of a collection of gas particles is directly proportional to absolute temperature only. (1) the gas is composed of a large number of identical molecules moving in random directions, separated by distances that are. The average kinetic energy of a collection of gas particles is directly proportional to absolute temperature only.
Source: www.chegg.com
(b) are attracted to each other by strong forces. Which choice is not an assumption or result of the kinetic theory of gases? Based on the kinetic theory, which statement is true?
Source: www.chegg.com
According to kinetic molecular theory (kmt), which statement is true? (1) the gas is composed of a large number of identical molecules moving in random directions, separated by distances that are. (c) according to the kinetic molecular theory, when gas particles collide, they bounce off.
Source: www.chegg.com
Matter is made up of only charged particles. According to the kinetic molecular theory collisions between gas particles are perfectly elastic. B)gas molecules move rondmly in different directions,continuously colliding with.
Source: www.chegg.com
In the kinetic molecular theory of gas behavior, the assumption is made that gas molecules (a) move rapidly in random directions. A) the collisions of particles with one another is completely elastic b) the size of the particle is large compared to the volume c) a single particle does not move in a straight line d) the average kinetic energy.
Source: www.chegg.com
Molecules of different gases with the same mass and temperature always have the same average volume. All particles have energy, but the energy varies depending on the temperature the sample of matter is in. Quesliun which statement is true about kinetic molecular theory?
Source: www.chegg.com
Molecules of different gases with the same mass and temperature always have the same average density. Kinetic molecular theory states that an increase in temperature raises the average kinetic energy of the molecules. The particles of matter are in constant motion.
Source: www.numerade.com
A single particle does not move in a straight line. Kinetic molecular theory states that gas particles are in constant motion and exhibit perfectly elastic collisions. In the kinetic molecular theory of gas behavior, the assumption is made that gas molecules (a) move rapidly in random directions.
Source: www.clutchprep.com
The particles of matter have zero kinetic energy. (4 points) matter consists of only large molecules. Based on the kinetic theory, which statement is true?
Source: www.clutchprep.com
Kinetic molecular theory states that an increase in temperature raises the average kinetic energy of the molecules. The kinetic energy of an object is the energy it has because of its motion. Kinetic molecular theory states that gas particles are in constant motion and exhibit perfectly elastic collisions.